Experiment Report of Pancreatic Cancer 2022-11-23
Pancreatic Cancer Efficacy Evaluation Experiment Results
In 2018, pancreatic cancer caused approximately 432,242 deaths worldwide, it's estimated the number of deaths is about 456,280 worldwide in 2020 and the estimated number of deaths is about 777,423 worldwide in 2040 by WHO. According to the American Cancer Society's estimates for pancreatic cancer in the United States for 2020, about 57,600 people will be diagnosed with pancreatic cancer and about 47,050 people will die of pancreatic cancer. Therefore, major pharmaceutical companies or government agencies worldwide have committed to developing new drugs aimed at increasing the survival rate of pancreatic cancer patients. However, most of the drugs not only have many serious side effects but also the patient's survival rate has not improved significantly.
However, the products we have developed for more than ten years may rekindle hope for pancreatic cancer patients.
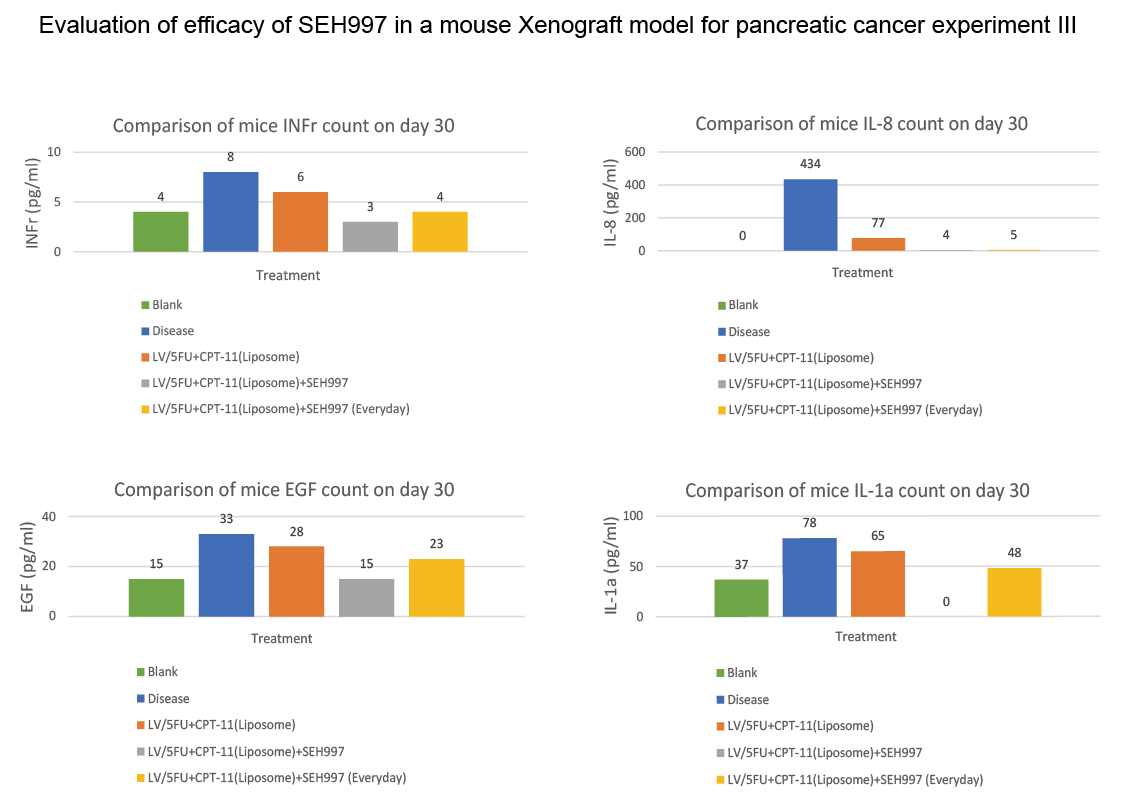
SEH® 997 concentrated solution has passed various animal tests by the Development Center for Biotechnology (DCB), Taiwan, which is a government grants organization whose managing director is the President of Academia Sinica, Taiwan. The DCB conducted animal studies wherein mice were administered with human MIA-PaCa2 pancreatic cancer cells, followed by chemotherapy for two weeks and observation after two weeks. It was confirmed that the mice received current chemotherapy drugs such as “LV/5FU+ CPT11 (Liposome) to simulate the leading Pancreatic and Colorectal cancer drug “ ONIVYDE®, which has been listed as the standard treatment for the pancreatic cancer patient group by the European Cancer Treatment Guidelines (ESMO Clinical Practice Guidelines, 2015) and the US Cancer Treatment Guidelines (NCCN Clinical Practice Guidelines, 2016). The result SEH®997 was combined with LV/5FU+CPT11(liposome) the tumor volume inhibition rate from 76% increase to 88% in 30 days. The Proinflammatory Cytokine IL-8 decreased from 77 pg/ml to 4 pg/ml (Blank 0 pg/ml and Disease 434 pg/ml). (Experiment I)
At present, the first-line treatment combination for pancreatic cancer in Taiwan is Leucovorin (LV)+Gemcitabine (GEM)+TS1; while in the United States, it is GEM +Abraxane (Abrax). However, the latest FDA-approved drug in the United States, "ONIVYDE®", which is recognized worldwide and uses the chemotherapy drug, CPT11 with liposome and adjuncts to 5FU + LV, has three months prolonged survival period, reaching an average of about 13 months as compared to patients who received GEM+Abrax, which is the first-line treatment combination for pancreatic cancer in the United States.
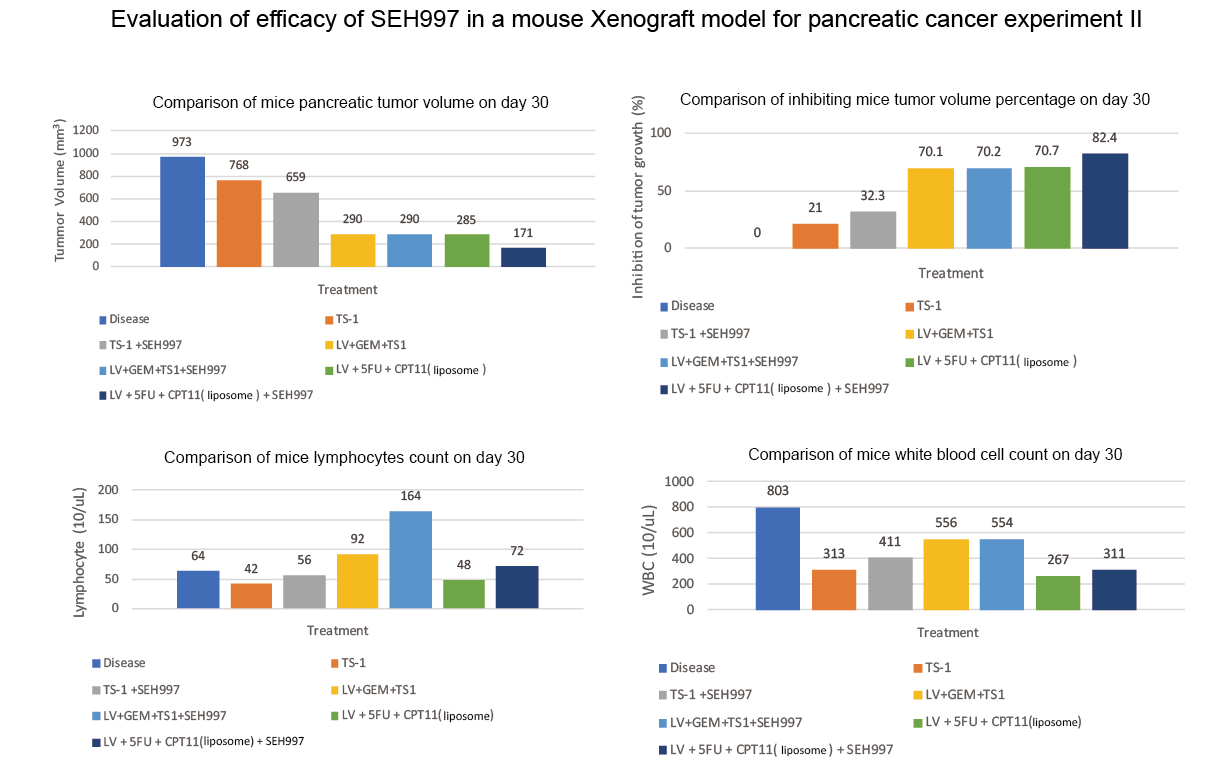
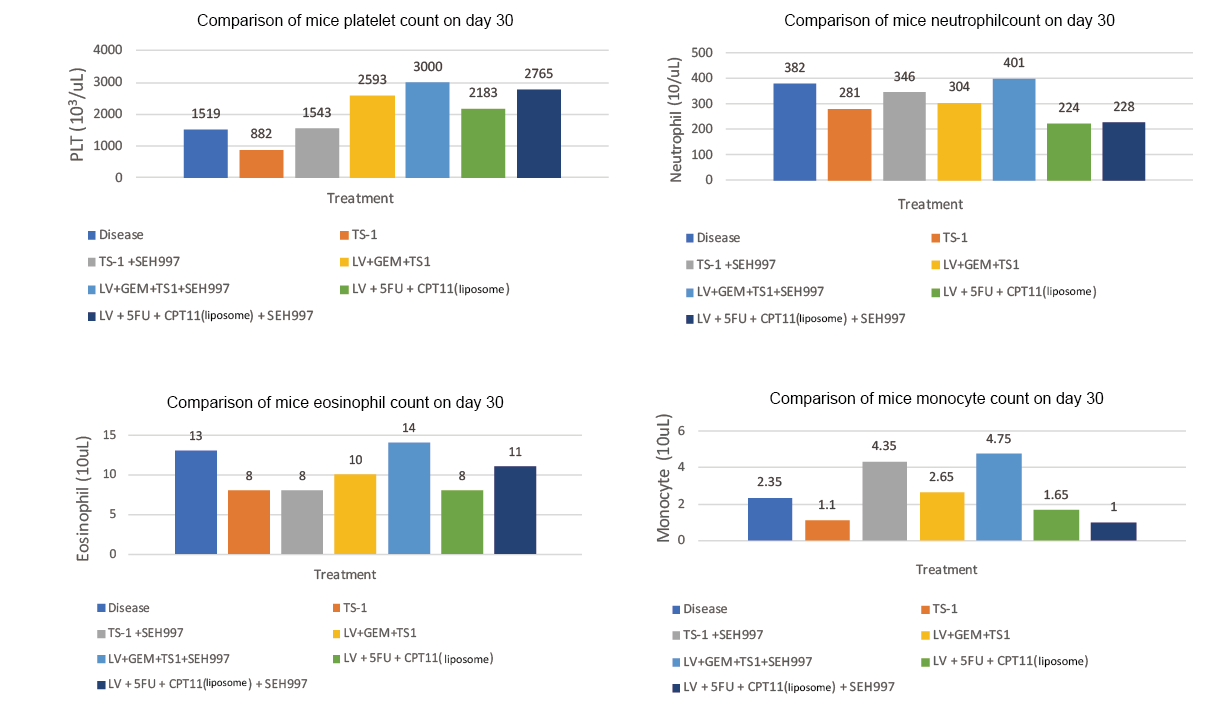
This animal study used the combination of ONIVYDE® as chemotherapy for pancreatic cancer, LV/5FU+CPT11 (with liposome), and adjunct to SEH®997 Concentrated Solution. The study results confirmed that after adding SEH®997 Concentrated Solution, the tumor inhibition rate increased from 70.7% to 82.4%, with 50% more lymphocytes, an increase of 17% white blood cells, and 27% more platelets, as compared to the group that received the combination of ONIVYDE® chemotherapy alone. (Experiment II)
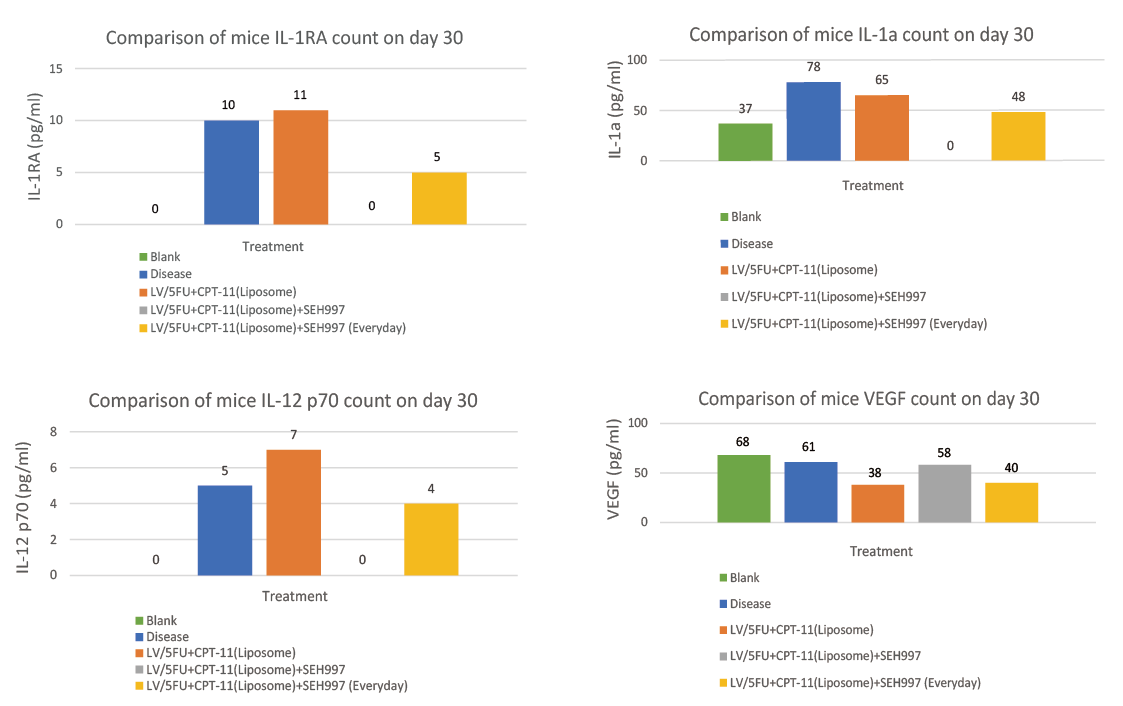
In Experiment III, the performance of the mice cytokines (including INFr, EGF, IL-8, IL-1a, IL-1RA, IL-12 p40, IL-12 p70, and VEGF) showed that, compared with using ONIVYDE® alone, in the combination of using SEH®997 reduced the degree of inflammation of IL-1a from 65pg/ml to 0pg/ml (control group 37pg/ml and disease group 78pg/ml), IL-1RA decreased from 11pg/ml To 0pg/ml (control group 0pg/ml and disease group 10pg/ml), IL-12 p40 decreased from 121pg/ml to 38pg/ml (control group 37pg/ml and disease group 115pg/ml).
The data from skipping SEH®997 on chemotherapy day (Group 4) and using SEH®997 every day (Group 5) showed that the degree of inflammation of cytokine in skipping SEH®997 on chemotherapy day is much lower. And it's best to skip using SEH®997 on the day of chemotherapy, which can maximize the effect of chemotherapy drugs. (Experiment III)
There was liver cell damage that caused lethal hepatic necrosis when using the chemotherapy drug, 5FU in the ONIVYDE® combination as chemotherapy for pancreatic cancer, while a previous animal study of colon cancer conducted by the DCB wherein mice were administered with 5FU and SEH®997 Concentrated Solution, demonstrated liver protection function.
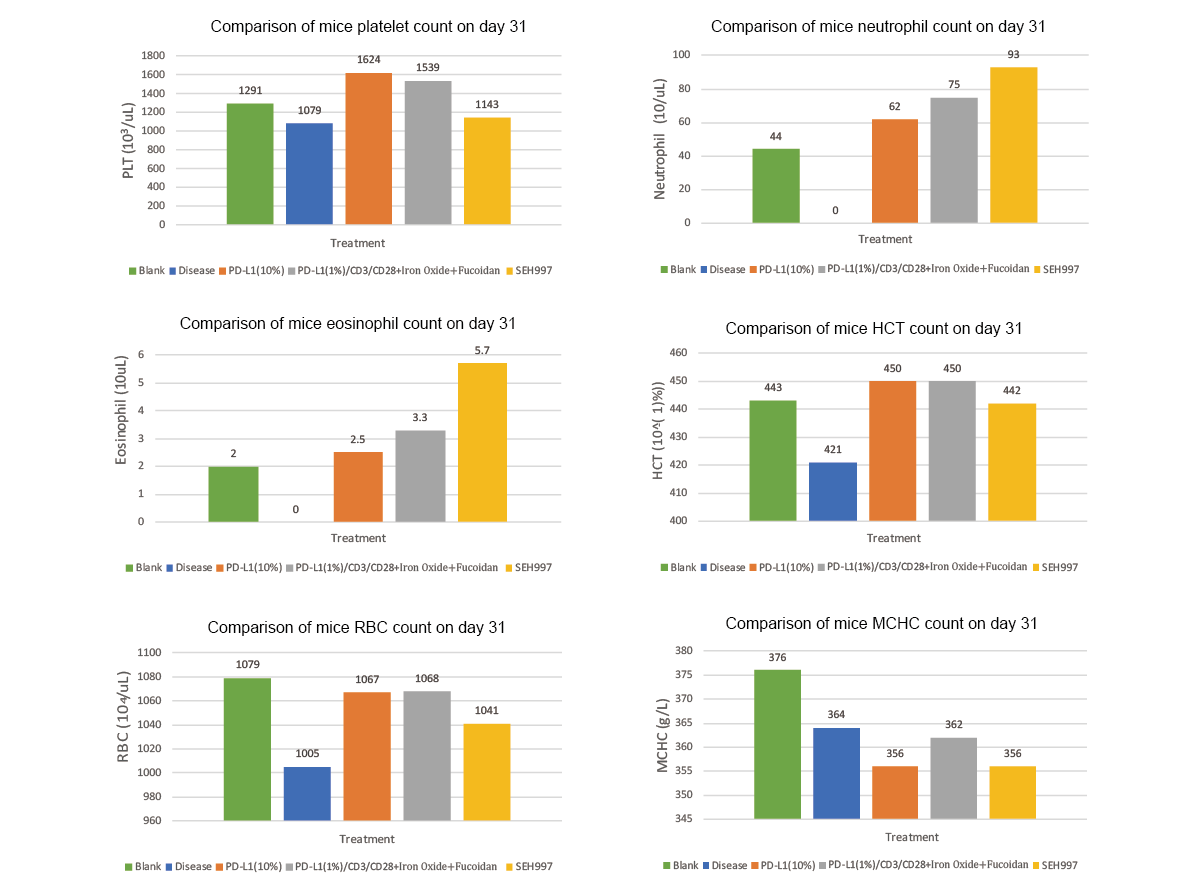
Something more worth mentioning, in our latest research in mouse models, we observed for the first time that the group treated with SEH997 only had significantly lower pancreatic tumor development than the disease group (p<0.01) and further indicated an increased tumor volume growth inhibition rate 34.2% in 31 days, which is compared with 32% by using PD-L1(10%) and 21% by using L1(1%)/CD3/CD28+IIron Oxide+Fucoidan. (Experiment IV)
Each consumption of SEH®997 is 10 drops (about 0.5cc) and must be diluted to 200-300cc of water. The total daily consumption is 30 drops to 50 drops (about 1.5 to 2.5 cc), which can be consumed 3 to 5 times a day. Also, SEH®997 better be skipped during the day of chemotherapy to get the best treatment result.
Besides, to accomplish the Cisplatin-induced leucopenia model confirmed by DBC after mice were administered “Cisplatin” and “Cyclophosphamide”, administration of G-CSF white blood cell growth hormone for comparison with the SEH®997 concentrated solution. The experimental results show that the mice which had SEH®997 had better white blood cells and lymphocyte enhancement after administering the chemotherapy drugs, also the effect is better than administering the drug G-CSF which may have many side effects.
There are many other experiments for other cancer tests. In the animal tests of colorectal cancer, mice were injected with chemotherapy drugs, such as 5-Fluorouracil (5-FU) and Cisplatin. DCB lab confirmed the SEH® 997 Concentrated Solution can protect the liver function, and increase the White Blood Cell (WBC) numbers of mice by 1.8~3 times, Lymphocytes 1.2~4.7 times, Monocytes 1.8~11.3 times, and Granulocyte 2.1~3 times. Also, by using SEH® 997 with 5FU chemotherapy, tumors can be shrunk by 4-6 times compared with using 5FU chemotherapy alone.
Meanwhile, DCB also confirmed when mice were injected with the Taxol chemotherapy drug, SEH® 997 can minimize the side effect such as arrhythmias leading to cardiac hypertrophy, which may cause an increase in organ weights, such as heart, liver, spleen, and kidney, while relieving pain caused by Taxol. Also, in the Cytokine experiments, DCB confirmed SEH® 997 concentrated solution has an effect of decreasing expression of human IL-8, IL-6, IL-1RA TNF-α Cytokine and suppressing mouse G-CSF(p<0.05), IL-6, IL-12(p70) and human IL-12(p70) Cytokine expression.